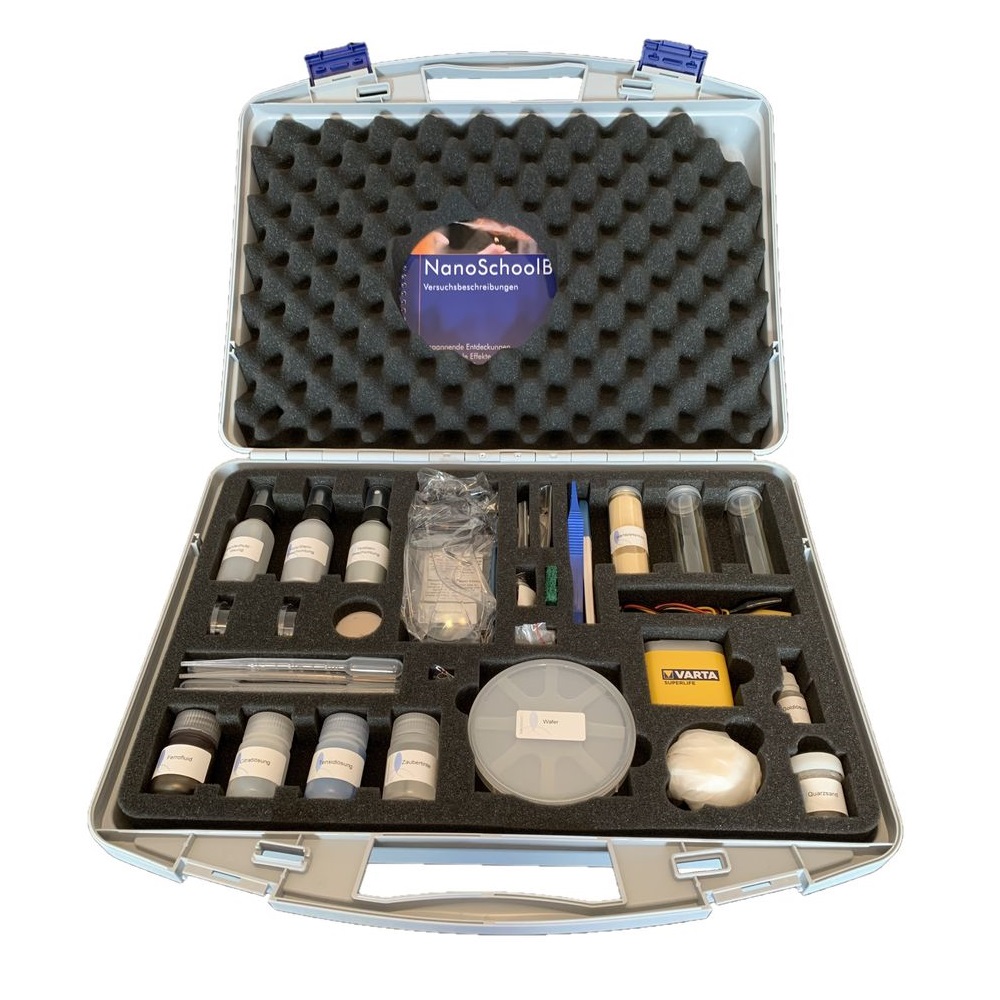
Nano SchoolBox 2.0
370.00€
Get ready for NanoSchoolBox 2.0.
Items in the kit:
1.Fire Protection sprey
2. Wood surface
3. textile
4. Protective glasses
5. Memory wire
6. Paint brush
7. ITO glass slide
8. Glass slide
9. Plastic pincers
10. pregnacy test
11. Lycopodium spores
12. snap cap glass container
13. perti dish
14. wax
15. LED
16. Abrassive sponge
17. Aligator cables
18. crystal rock
19. pipettes
20. magnet
21. wood floor
22. Battery
23. Auric chloride
24. Ferrofluid
25. citrate solution
26. tenside solution
27. invisible ink
28. silicon waffer
29. protective gloves
30. Silica sand
These materials are sufficient for 15 experiments and for 4 display experiments. The display items are:
Pregnancy test
Silica sand
Crystal rock
Silicon waffer
The experiments can be performed either by teachers or by the students themselves in physics, biology or chemistry classes.
It is also ideal for demonstrating experiments at universities, conferences and scientific festivals.
The experiments
• Experiments to obtain the lotus effect
• Hydrophobic surfaces for dummies
• Hydrophobic surfaces on wood and stone
• Hydrophobic surfaces on textiles
• Hydrophilic surfaces: “invisible ink” on glass
• Scratch-resistant coating on wood
• Nanocoating for fire protection
• Electrical conductivity of glass through indium tin oxide
• Photocatalysis with titanium oxide
• Ferrofluids in the magnetic field
• Density separation with ferrofluid
• Identification of colloids through the Tyndall effect
• Production of nanoscale gold
• Memory metal – atom movements in the nanodimension
• Spitting fire with small particles
• Superhydrophobia
The demo objects
• Pregnancy test: gold particles identify biomolecules
• From sand to chip I: silica sand
• From sand to chip II: rock crystal
• From sand to chip III: silicon wafer
In recent decades, a new interdisciplinary science has developed from the fields of physics, chemistry, biology, medicine, and the science of materials: nanotechnology. Its goal is to make nanoscale processes and components usable in scientific and industrial applications by technical means.
It is noteworthy that these materials are bio-mimetic materials. In other words, these are materials that mimic, copy, or learn from nature. Many of the properties considered, such as changing our hydrophilicity or surface resistance, come from properties already found in nature. This technology utilizes the knowledge of nature to improve the properties of materials used by humans.
Detailed experiments:
Experiment 1: Experiments to achieve the “natural cleansing” of the lotus?
The aim of this experiment is to determine whether certain materials have a hydrophobic surface. Hydrophilic fibers on some of the surface of the leaves exert adhesive forces on the water, which increase the larger their surface area (or the harder the surface of the paper). For this reason, the water drop spreads on the surface to a greater or lesser degree. In nature, this natural cleansing effect is responsible for the surface of the leaf that remains mostly clean.
Experiment 2: Hydrophobic artificial surfaces
Along with the natural lotus phenomenon found in the plant and animal kingdom, one can apply a kind of “lotus effect” to almost any type of artificial surface, ie a water repellent, hydrophobic effect. This should not always be based on a microstructure, as the hydrophobic properties can be added to surfaces by applying a coating containing fluorinated compounds, a Teflon-like effect. It should be noted, however, that a real lotus surface is always based on microstructures. Only the visible phenomenon is the same.
Experiment 3: Production of hydrophobic surface on wood or mineral material
This experiment involves the development of artificial hydrophobic surfaces in materials such as wood or stone (you will do hydrophobic [water repellent] treatment.
Experiment 4: Production of hydrophobic surface on fabrics
As in the previous experiment, you will develop a hydrophobic effect in this experiment as well. It is based on the same principle as Experiment 2. However, in this case you are dealing with a special impregnation to obtain a hydrophobic and oil-repellent (oil repellent) for fabrics and paper.
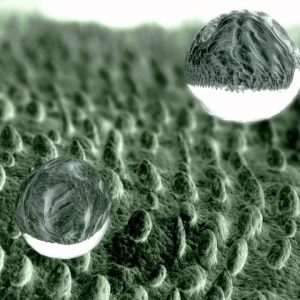

Experiment 5: Invisible ink for glass – production of hydrophilic surface (anti-fog)
Now you will learn how to develop the opposite of the hydrophobic effect, ie the hydrophilic (water-friendly) effect. What is so special about a surface that has been treated with the so-called “anti-fog coating” is the fact that it does not absorb water but that the aqueous solutions are “spread” (ie dispersed). Instead of seeing a drop, we see nothing. In this experiment we process a glass surface with a hydrophilic agent to write a hidden message on it.
Experiment 6: Scratch-resistant wood coating
In general, MDF panels (medium density fiberboard) are used to produce interior doors, front and rear pieces of furniture with wooden designs. Designs are printed using a variety of printing techniques, such as inkjet printing on panels. One problem that is often associated with this process is that the surface is particularly sensitive to mechanical stress and can therefore be easily scratched.
In order to protect the designs on the MDF panels, a transparent, thin 10 mm protective coating was applied, in which nanoparticles were incorporated. This gives the panels more stability. Even if we scratch the surface with a wire brush, the panel will not scratch.

Experiment 7: Fire protection
Paper, cardboard or wood are mostly made up of cellulose, a carbohydrate. When the solution is poured onto the paper and heated, the phosphorite is chemically combined with the carbohydrate and converted to carbon, but without burning in the process. Phosphoric acid esters are formed, which decompose under heat to become phosphoric acid and carbon. Nitrogen compounds in this case have the following function:
heat causes the formation of nitrogen gas (which in itself is non-flammable) and displaces the oxygen required at the source of the fire (there is no flame without oxygen).
Experiment 8: Increase electrical conductivity through the cathode (ITO) oxide
Common glass is not conductive, ie it cannot transmit electric currents. However, the special glass in this box can do this because the glass is covered with an extremely thin, invisible nanostrate, the so-called ITO layer. The term ITO means Indian-Cash Oxide, a compound of mixed Indian oxide and tin oxide. It is semi-conductive and transparent, these properties form the basis for the production of electrically conductive glass.
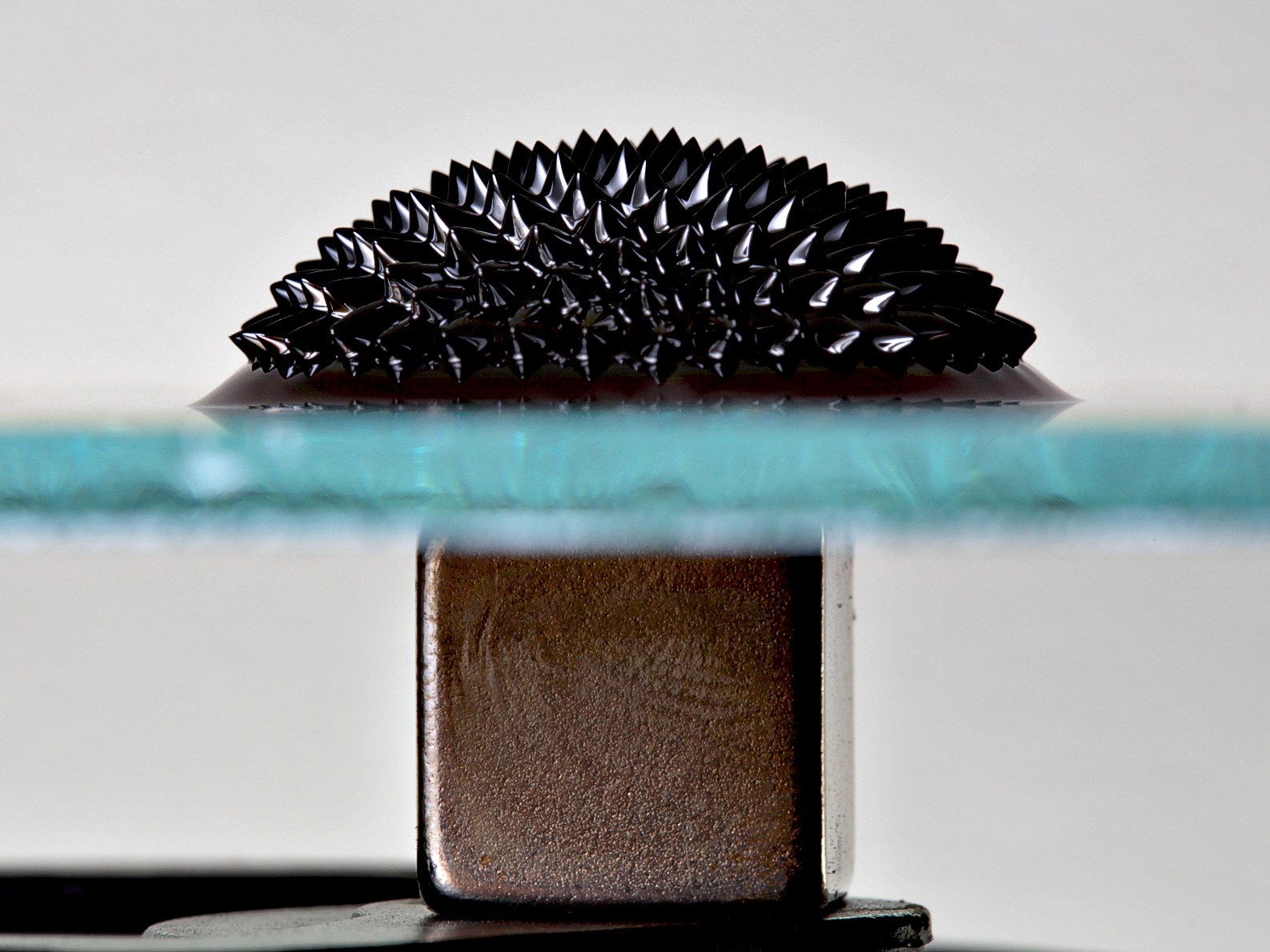
Experiment 9: The magnetic fieldWith the help of iron fillers, it is very easy to prove the magnetic field around a magnet, the field that pulls from the north to the south pole of a magnet and that forms closed circuits. The irons are aligned according to the power lines and try to follow them.
Experiment 10: density separation with ferrofluid
In this experiment, a non-magnetic coin, which first sinks to the bottom of the liquid due to its density, “swims” to the top in the presence of a magnetic field.
Experiment 11: Recognition of colloids through the Tyndall phenomenon
Using fairly simple methods you will see how the nanoclimate, colloidal systems in liquids can be detected through the Tyndall effect.
Experiment 12: Gold nanoparticle production
In the following experiment, we will produce clusters of golden nanoscale that will be easily recognizable through the manifestation of a typical red ruby color.
One way to produce gold nanoparticles is through the citrate method. This method involves the production of colloidal gold or gold clusters in a solution.
The experiment is based on an oxidation-reduction reaction of tetrachloroacrylic acid (known as tetrachloroacetic acid (III) trihydrate) in which gold ions are reduced to clusters of gold atoms. Sodium citrate reducer not only serves to reduce gold, but also acts as a diluent to stabilize the created gold clusters. With the addition of the reducer, the coagulation of the metal ions stops. The result is a colloidal complex enclosed by a binder.
Experiment 13: Memory metal – movements of atoms in the nanostructure. This experiment involves NITINOL wire. NITINOL is the name given to almost all the stoichiometric compositions consisting of nickel and titanium that display the so-called memory effect. The wire used in this experiment has pseudo-elastic behavior.
Experiment 14: Fire spitting from small particles. The lycopodium (also known by the common name wolf foot or fox tail) is a plant belonging to the genus Cryptogamous. In the past, lycopene seeds (seeds from lycopodium clavatum) were used for medicinal purposes to dry wounds (which is why it is also called the witch’s flour ).Today, lycopodium seeds are used in pyrotechnic products and are used in forensic science to detect fingerprints (in combination with other substances).
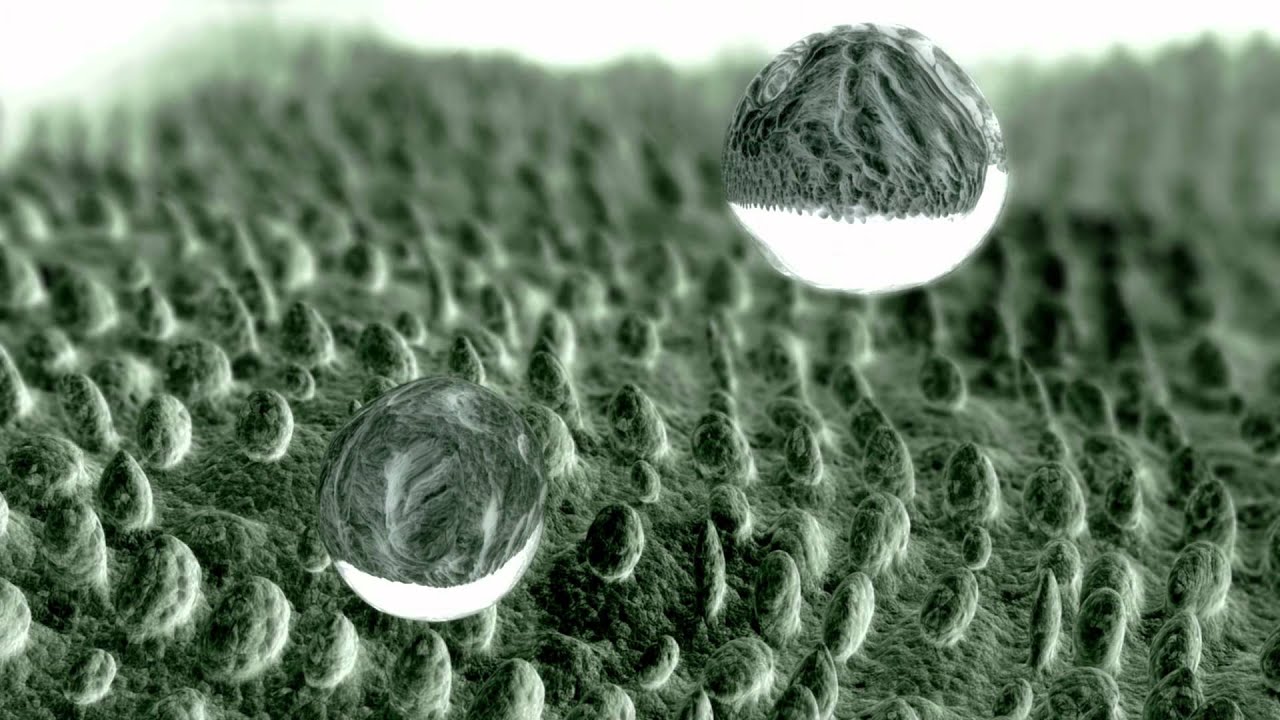
Experiment 15: Hyperhydrophobia. A surface is considered hydrophobic when the contact angle between the water droplets and the surface exceeds 90 degrees. A super-hydrophobic surface is one in which the contact angle exceeds 150 degrees. A surface becomes more hydrophobic as it becomesrougher. Such surfaces are also known as freckles surfaces, as the drop is like a freckle on a nail bed, which comes in contact with the surface only in certain places. Air pockets are formed between the contact points on these surfaces, which further reduces adhesion.
The kit also includes 4 display items:
- Pregnancy test
- Silica sand
- Crystal rock
- Silicon waffer










 STEMATHLON
STEMATHLON